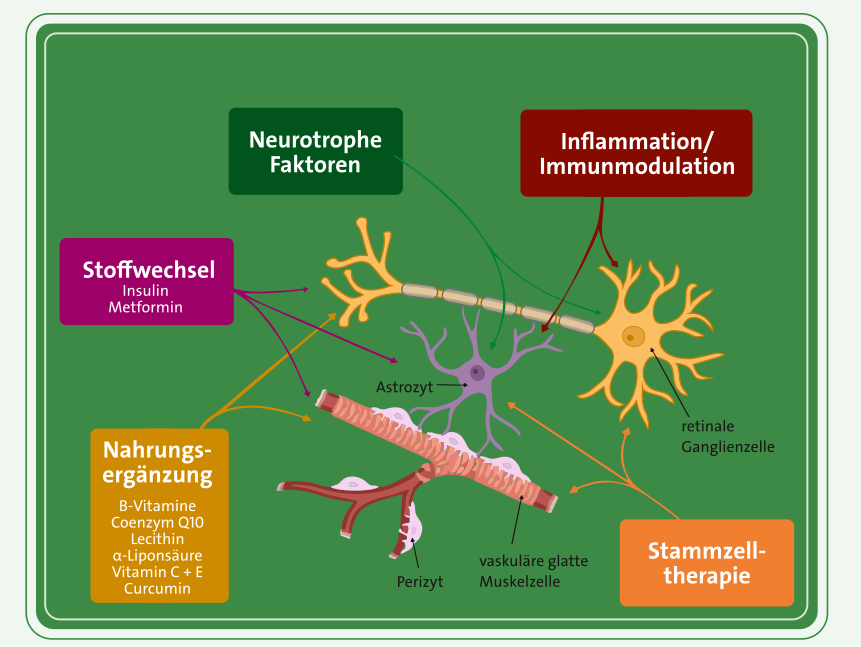
Neuste Glaukom-Forschung konzentriert sich auf die Thematik Neurodegeneration und sucht Wege, um dieser effektiv entgegenzuwirken.
„Das weite Feld der Neuroprotektion im Rahmen der Glaukomerkrankung zeigt mit der Entwicklung von neurotrophen, antioxidativen, antiexzitotoxischen, antiischämischen, antiinflammatorischen, antiapoptotischen und immunmodulatorischen Therapieansätzen vielversprechende Fortschritte, die Neurodegeneration zu vermindern und somit die Sehfunktion zu stabilisieren.“
Prof. Dr. med. Carl Erb
In diesem Beitrag finden Sie wichtige Forschungsbeiträge. Klicken Sie sich durch die Übersicht oder laden Sie direkt eine Komposition sämtlicher Abstracts hier herunter:
Veröffentlicht in: The Ophthalmologist (2024, April 7)
Link: https://theophthalmologist.com/subspecialties/neuroprotection-the-future-of-glaucoma-treatment
Forschungsteam: Bou Ghanem, G. O., Calkins, D. J.
Abstract: Current glaucoma treatments focus on lowering intraocular pressure (IOP), which is akin to fighting a raging fire with a bucket of water – the intervention slows the inferno, but it cannot extinguish it. For many patients, there simply are not enough „buckets.” And, like many other researchers, we share the realization that more must be done.
The truth is this: our single-minded pursuit of managing IOP is failing a significant portion of patients. Glaucoma progressed in 20 percent of participants in a UK-based glaucoma treatment study (UKGTS) by 24 months, and an earlier study revealed that 46 percent of patients receiving IOP-lowering surgery progressed to blindness within a decade, even when IOP was “controlled.” Patients need a paradigm shift – a multipronged strategy that not only targets IOP management but also actively supports, protects, and even repairs RGCs and the optic nerve.
We want to create glaucoma treatments that do more than lower pressure; we want to empower RGCs to fight back. This goal demands a greater understanding of the relationship between the mechanical stress, vascular and metabolic dysfunction, and inflammatory cascades that contribute to glaucomatous damage to the optic projection. Think of it as equipping the system and its constitutive elements (RGCs, axons, glia, vessels, extracellular matrix, and so on) with a comprehensive toolkit to manage oxidative stress, optimize energy production, and mitigate inflammation. The good news is that neurons are inherently resilient fighters, with adaptive responses in their arsenal. It is important to note, though, that these responses are limited by resource constraints and require external support for long-term survival and recovery.
One field that could have a positive impact in this regard is neuroprotection or neurorepair. This approach has the capability to combine diverse strategies, each targeting a specific aspect of the disease during a specific point in progression, to create a personalized treatment regimen for each patient or for each type of glaucoma. But it hinges on early detection. The sooner we intervene, before irreversible damage sets in, the greater the chance of success. New biomarkers – molecular, electrophysiological, and imaging – are of particular interest, as they offer earlier and more accurate detection. Other testing methods, like clustered testing, are an additional way we can quickly identify eyes with rapidly progressing glaucoma, thus improving the feasibility of glaucoma clinical trials.
The road to a combination neuroprotective future is not without its bumps, however. Combination therapy trials are notoriously difficult and expensive. They will require adaptive trial designs that adjust based on interim data analysis, as well as robust collaborations between researchers, industry, and regulatory bodies. The validity of preclinical models is also crucial. No single model can perfectly capture the intricate complexities of glaucoma. Therefore, evaluating treatment regimens in a battery of diverse experimental models, including non-human primates with their close resemblance to human ocular anatomy, is paramount. Only through this multifaceted approach can we establish robust evidence for the effectiveness of combination therapies, setting a foundation for evaluation in clinical trials.
The future of glaucoma treatment is not about a single magic bullet but rather a well-equipped and diverse arsenal. Through the power of neuroprotection, early detection, and combination therapy, we can rewrite the narrative against this condition – one that moves beyond the limitations of IOP-centric approaches.
Veröffentlicht in: Progress in Retinal and Eye Research, 100, 101261 (2024)
Link: https://pubmed.ncbi.nlm.nih.gov/38527623/
Forschungsteam: Bou Ghanem, G. O., Wareham, L. K., Calkins, D. J.
Abstract: Glaucoma is the leading cause of irreversible blindness globally, resulting in vision loss due to the neurodegeneration of the retinal ganglion cell (RGC) projection to the brain through the optic nerve. It is associated with sensitivity to intraocular pressure (IOP), and while treatments primarily focus on managing IOP, many patients still experience vision loss. To address neurodegeneration directly, numerous preclinical studies are exploring protective or reparative therapies that act independently of IOP, including growth factors, metabolic compounds, anti-inflammatory and antioxidant agents, and neuromodulators. Despite promising results in experimental models, many of these approaches fail to translate into clinical benefits. Several factors contribute to this challenge, such as differences in the anatomical structure of the optic nerve head between rodents, nonhuman primates, and humans, and the inability of animal models to fully replicate the complex pathophysiology of glaucoma in humans. To improve the success of translating these findings, we propose two approaches: first, thorough evaluation of experimental targets in multiple animal models, including nonhuman primates, before proceeding to clinical trials; and second, the use of combination therapy, which involves applying multiple agents simultaneously, particularly in the early and potentially reversible stages of the disease. These strategies aim to increase the likelihood of successful neuroprotective treatments for glaucoma.
Veröffentlicht in: Klinische Monatsblätter Für Augenheilkunde, 237(02), 163–174. (2020)
Link: https://www.thieme-connect.com/products/ejournals/abstract/10.1055/a-1093-0945
Forschungsteam: C. Erb
Abstract: Primary open-angle glaucoma (POAG) is currently considered a neurodegenerative systemic disease, and the individualized reduction of intraocular pressure alone does not adequately address the complex events involved in the chronic course of the disease, making accompanying drug therapy concepts useful. Four therapeutic strategies are highlighted: improving the mitochondrial respiratory chain with coenzyme Q10, stabilizing the mitochondrial and cellular plasma membranes with citicoline, reducing oxidative stress in the eye and body with curcumin, and improving fat metabolism, particularly LDL cholesterol, through the application of statins. These approaches provide insight into potential additive therapeutic options. Since neuroprotective therapies are unlikely to be highly effective as monotherapies, they are more likely to consist of a combination of different therapeutic approaches.
Veröffentlicht in: Der Ophthalmologe, 118(5), 439–448 (2021)
Link: https://link.springer.com/article/10.1007/s00347-021-01362-z
Forschungsteam: Jünemann, A. G. M., Grieb, P., Rejdak, R.
Abstract:
Hintergrund: In den letzten Jahren wurde in zahlreichen experimentellen und klinischen Studien gezeigt, dass die neuronale Degeneration bei der Glaukomerkrankung nicht nur auf der Ebene der Netzhaut und des Sehnervs, sondern entlang der gesamten Sehbahn und im Gehirn auftritt. Material und
Methode: Vor dem Hintergrund der Neuroanatomie, des Neuroimaging und der Pathogenese des Glaukoms wird die Relevanz dieser Erkenntnisse erläutert. Die Daten aus experimentellen und klinischen Studien werden dargestellt und ein Fazit für die klinische Anwendung gezogen.
Fragestellung: Die neuroprotektive Wirkung von Citicolin bei Glaukom und die dahinterliegenden Mechanismen werden beschrieben.
Schlussfolgerung: Citicolin wirkt neuroprotektiv über glaukomrelevante Mechanismen, wobei die neuroprotektive Wirkung bei Offenwinkelglaukomen sowohl funktionell als auch morphologisch nachweisbar ist. Diese Wirkung ist unabhängig vom Glaukomschaden und Augeninnendruck und tritt in der Regel erst nach einem Jahr auf. Die Effekte von oralem Citicolin zeigen sich bei einer Tagesdosis von 500–1000 mg, und die Einnahme kann dauerhaft oder in Zyklen erfolgen. In den Studien wurden bei der Einnahme von Citicolin keine Nebenwirkungen festgestellt. Zudem kann Citicolin kognitive Leistungen, die Therapieadhärenz und die Lebensqualität bei Glaukompatienten verbessern. Diese relativ alte nootrope Substanz, die heute als Nahrungsergänzungsmittel vermarktet wird, scheint eine wertvolle Ergänzung zur konventionellen Therapie der Glaukomerkrankung sowie eine rationale Option zur Neuroprotektion und Prophylaxe darzustellen.
Veröffentlicht in: Neuropharmacology, 43(6), 1015–1025 (2002)
Forschungsteam: Chidlow, G., Schmidt, K.-G., Wood, J. P. M., Melena, J., Osborne, N. N.
Abstract: The aim of this study was to examine whether the antioxidant α-lipoic acid protects retinal neurons from ischemia-reperfusion injury. Rats were injected intraperitoneally with either vehicle or α-lipoic acid (100 mg/kg) once daily for 11 days. On the third day, ischemia was delivered to the rat retina by raising the intraocular pressure above systolic blood pressure for 45 minutes. The electroretinogram was measured prior to ischemia and 5 days after reperfusion. Rats were killed 5 or 8 days after reperfusion, and the retinas were processed for immunohistochemistry and for determination of mRNA levels by RT-PCR. Ischemia-reperfusion caused a significant reduction of the a- and b-wave amplitudes of the electroretinogram, a decrease in nitric oxide synthase and Thy-1 immunoreactivities, a decrease of retinal ganglion cell-specific mRNAs, and an increase in bFGF and CNTF mRNA levels. All of these changes were clearly counteracted by α-lipoic acid. Moreover, in mixed rat retinal cultures, α-lipoic acid partially counteracted the loss of GABA-immunoreactive neurons induced by anoxia. The results of the study demonstrate that α-lipoic acid provides protection to the retina as a whole, and to ganglion cells in particular, from ischemia-reperfusion injuries. α-lipoic acid also displayed negligible affinity for voltage-dependent sodium and calcium channels.
Veröffentlicht in: Investigative Ophthalmology and Visual Science, 56(11), 6638–6645 (2015)
Link: https://iovs.arvojournals.org/article.aspx?articleid=2463756
Forschungsteam: Xia, H., Nan, Y., Huang, X., Gao, J., Pu, M.
Abstract:
Purpose: To investigate the effects of tauroursodeoxycholic acid (TUDCA) and alpha-lipoic acid (ALA) on the visual response properties of cat retinal ganglion cells (RGCs) in wholemount retinas.
Methods: Young adult cats were divided into three groups: control, ALA, and TUDCA. In vitro single-unit extracellular recordings were performed on wholemount retinas to objectively evaluate the visual response properties of RGCs prior and post to antioxidant treatment. The visual response properties of RGCs, including receptive field size, luminance threshold, and contrast sensitivity, were collected online and analyzed off-line with Axon Pclamp9.
Results: Most of the RF sizes were larger than those plotted prior to the 60 minutes dark adaptation. The luminance threshold was elevated in the control group (no treatment) but reduced post ALA treatment and significantly reduced post TUDCA treatment. The contrast threshold was significantly elevated in the control group (no treatment) and clearly elevated post ALA treatment but effectively sustained post TUDCA treatment.
Conclusions: Retinal neurocircuitry deteriorates in wholemount retinas, resulting in abnormal visual response properties in RGCs. Alpha-lipoic acid and TUDCA exerted beneficial neuroprotective effects by activating the antioxidant pathway, partially restoring the functionality of retinal neurocircuitry and significantly improving the visual response properties of RGCs. However, TUDCA appears to be more effective than ALA in reducing irradiance thresholds and improving contrast sensitivity.
Veröffentlicht in: Experimental Brain Research. Experimentelle Hirnforschung. Expérimentation Cérébrale, 167(4), 641–648 (2005)
Link: https://link.springer.com/article/10.1007/s00221-005-0068-0
Forschungsteam: Matteucci, A., Frank, C., Domenici, M. R., Balduzzi, M., Paradisi, S., Carnovale-Scalzo, G., Scorcia, G., Malchiodi-Albedi, F.
Abstract: Curcumin, an extract from the plant Curcuma longa with well-known antioxidant and anti-inflammatory activities, was tested as protective agent against excitotoxicity in rat retinal cultures. A 24 h-treatment with curcumin reduced N-methyl-D: -aspartate (NMDA)-mediated excitotoxic cell damage, estimated as decrease of cell viability and increase in apoptosis. The protection was associated with decrease of NMDA receptor-mediated Ca(2+) rise and reduction in the level of phosphorylated NR1 subunit of the NMDA receptor. These results enlighten a new pharmacological action of the plant extract, possibly mediated by a modulation of Experimental Brain Research.
Veröffentlicht in: Journal of Ocular Pharmacology and Therapeutics, 30(8), 657–664 (2014)
Link: https://www.liebertpub.com/doi/10.1089/jop.2014.0022
Forschungsteam: Matteucci, A., Frank, C., Domenici, M. R., Balduzzi, M., Paradisi, S., Carnovale-Scalzo, G., Scorcia, G., Malchiodi-Albedi, F.
Abstract:
Methods: BV-2 microglia cell line was used in an in vitro study and Wistar rats were used in an in vivo study. Cultured BV-2 microglia cells were pretreated with 10, 1, or 0.1 μM curcumin for 1 h, and sustained oxidative stress was induced by subjecting BV-2 microglia to 200 μM hydrogen peroxide (H2O2) for 24 h. MTT assay was used to determine cell viability. Changes of intracellular reactive oxygen species (ROS) and apoptosis were analyzed by flow cytometry. Three episcleral veins were cauterized to induce high IOP in Wistar rats and measured by Tonopen. After 6 weeks of treatment with curcumin (10 mg/kg/day) by intragastric administration, surviving retinal ganglion cells were quantified. Activation of caspase 3, cytochrome c, BAX, and BCL2 was quantified by Western blotting both in BV-2 microglia and in the animal model. Data were analyzed with the GraphPad Prism 5.0 software, and P<0.05 was considered to be statistically significant.
Results: The in vitro study showed that when BV-2 microglia was pretreated with curcumin, the cell viability increased and the intracellular ROS and apoptosis significantly decreased. In the in vivo study, chronic mild IOP elevation was induced for 4 weeks. In the curcumin-treated group, curcumin protected rat BV-2 microglia from death significantly. In both H2O2-treated BV-2 microglia and glaucoma models, caspase 3, cytochrome c, and BAX were downregulated and BCL2 was upregulated in the curcumin-treated group.
Purpose: The involvement of local and systemic oxidative stress in intraocular pressure (IOP) elevation and optic nerve damage has been hypothesized in the pathogenesis of glaucoma. In this study, we aim to evaluate the antioxidant effects of curcumin in BV-2 microglia oxidative damage and assess its neuroprotective effects in a chronic high IOP rat model.
Conclusions: Curcumin affords neuroprotective effects by inhibiting oxidative damage and could be a new or adjunctive treatment for glaucoma.
Veröffentlicht in: Investigative Ophthalmology and Visual Science, 57(10), 4327–4332 (2016)
Link: https://iovs.arvojournals.org/article.aspx?articleid=2546828
Forschungsteam: Lin, C., Wu, X.
Abstract:
Methods:
Purpose: Glaucoma is closely linked with oxidative stress and inflammation, and difficult to treat. Its occurrence frequently is contributed by the failure of the trabecular meshwork (TM). Curcumin is known as an antioxidative and anti-inflammatory substance, possessing the potential to treat glaucoma.
Methods: Using TM cells as the in vitro model system, we investigated the effects of curcumin on oxidative stress-induced markers for TM impairments, including cell death, production of intracellular reactive oxygen species (iROS), induction of proinflammatory proteins, activation of senescence marker, accumulation of carbonylated proteins, and apoptotic cell numbers.
Results: Curcumin treatment protected TM cells against oxidative stress-induced cell death. Curcumin treatment at concentrations between 1 and 20 μM reduced the production of iROS in H2O2-exposed TM cells in a dose-dependent manner. Further studies demonstrated that curcumin treatment (20 μM) significantly inhibited proinflammatory factors, including IL-6, ELAM-1, IL-1α, and IL-8, whereas it decreased activities of senescence marker SA-β-gal, and lowered levels of carbonylated proteins and apoptotic cell numbers.
Conclusions: Curcumin is capable of protecting TM cells against oxidative stress, shedding new light on potential treatment for glaucoma.
Veröffentlicht in: Scientific Reports, 8(1) (2018)
Link: https://www.nature.com/articles/s41598-018-29393-8
Forschungsteam: Davis, B. M., Pahlitzsch, M., Guo, L., Balendra, S., Shah, P., Ravindran, N., Malaguarnera, G., Sisa, C., Shamsher, E., Hamze, H., Noor, A., Sornsute, A., Somavarapu, S., Cordeiro, M. F.
Abstract: Curcumin (1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5dione) is a polyphenol extracted from turmeric that has long been advocated for the treatment of a variety of conditions, including neurodegenerative and inflammatory disorders. Despite this promise, the clinical use of curcumin has been limited by the poor solubility and low bioavailability of this molecule. In this article, we describe a novel nanocarrier formulation comprising Pluronic-F127 stabilized D-α-Tocopherol polyethylene glycol 1000 succinate nanoparticles, which were used to successfully solubilize high concentrations (4.3 mg/mL) of curcumin. Characterization with x-ray diffraction and in vitro release assays localize curcumin to the nanocarrier interior, with each particle measuring <20 nm in diameter. Curcumin-loaded nanocarriers (CN) were found to significantly protect against cobalt chloride-induced hypoxia and glutamate-induced toxicity in vitro, with CN treatment significantly increasing R28 cell viability. Using established glaucoma-related in vivo models of ocular hypertension (OHT) and partial optic nerve transection (pONT), topical application of CN twice daily for three weeks significantly reduced retinal ganglion cell loss compared to controls. Collectively, these results suggest that our novel topical CN formulation has potential as an effective neuroprotective therapy in glaucoma and other eye diseases with neuronal pathology.
Veröffentlicht in: Investigative Ophthalmology and Visual Science, 53(13), 8295–8302 (2012)
Link: https://iovs.arvojournals.org/article.aspx?articleid=2165655
Forschungsteam: Lulli, M., Witort, E., Papucci, L., Torre, E., Schipani, C., Bergamini, C., Monte, M. D., Capaccioli, S.
Abstract:
Purpose: To evaluate if coenzyme Q10 (CoQ10) can protect retinal ganglion cells (RGCs) from apoptosis and, when instilled as eye drops on the cornea, if it can reach the retina and exert its antiapoptotic activity in this area in a mouse model of kainate (KA)-induced retinal damage.
Methods: Rat primary or cultured RGCs were subjected to glutamate (50 μM) or chemical hypoxia (Antimycin A, 200 μM) or serum withdrawal (FBS, 0.5%) in the presence or absence of CoQ10 (10 μM). Cell viability was evaluated by light microscopy and fluorescence-activated cell sorting analyses. Apoptosis was evaluated by caspase 3/7 activity and mitochondrion depolarization tetramethylrhodamine ethyl ester analysis. CoQ10 transfer to the retina following its instillation as eye drops on the cornea was quantified by HPLC. Retinal protection by CoQ10 (10 μM) eye drops instilled on the cornea was then evaluated in a mouse model of KA-induced excitotoxic retinal cell apoptosis by cleaved caspase 3 immunohistofluorescence, caspase 3/7 activity assays, and quantification of inhibition of RGC loss.
Results: CoQ10 significantly increased viable cells by preventing RGC apoptosis. Furthermore, when topically applied as eye drops to the cornea, it reached the retina, thus substantially increasing local CoQ10 concentration and protecting retinal layers from apoptosis.
Conclusions: The ability of CoQ10 eye drops to protect retinal cells from apoptosis in the mouse model of KA-induced retinal damage suggests that topical CoQ10 may be evaluated in designing therapies for treating apoptosis-driven retinopathies.
Veröffentlicht in: Investigative Ophthalmology and Visual Science, 52(7), 4263–4273 (2011)
Link: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3175980/
Forschungsteam: Burugula, B., Ganesh, B. S., Chintala, S. K.
Abstract:
Purpose: Staurosporine (SS) causes retinal ganglion cell (RGC) death in vivo, but the underlying mechanisms have been unclear. Since previous studies on RGC-5 cells indicated that SS induces cell death by elevating proteases, this study was undertaken to investigate whether SS induces RGC loss by elevating proteases in the retina, and curcumin prevents SS-mediated death of RGCs.
Methods: Transformed mouse retinal ganglion-like cells (RGC-5) were treated with 2.0 μM SS and various doses of curcumin. Two optimal doses of SS (12.5 and 100 nM) and curcumin (2.5 and 10 μM) were injected into the vitreous of C57BL/6 mice. Matrix metalloproteinase (MMP)-9, tissue plasminogen activator (tPA), and urokinase plasminogen activator (uPA) activities were assessed by zymography assays. Viability of RGC-5 cells was assessed by MTT assays. RGC and amacrine cell loss in vivo was assessed by immunostaining with Brn3a and ChAT antibodies, respectively. Frozen retinal cross sections were immunostained for nuclear factor-κB (NF-κB).
Results: Staurosporine induced uPA and tPA levels in RGC-5 cells, and MMP-9, uPA, and tPA levels in the retinas and promoted the death of RGC-5 cells in vitro and RGCs and amacrine cells in vivo. In contrast, curcumin attenuated RGC and amacrine cell loss, despite elevated levels of proteases. An NF-κB inhibitory peptide reversed curcumin-mediated protective effect on RGC-5 cells, but did not inhibit protease levels. Curcumin did not inhibit protease levels in vivo, but attenuated RGC and amacrine cell loss by restoring NF-κB expression.
Conclusions: The results show that curcumin attenuates RGC and amacrine cell death despite elevated levels of proteases and raises the possibility that it may be used as a plausible adjuvant therapeutic agent to prevent the loss of these cells in retinal degenerative conditions.
Veröffentlicht in: PLoS ONE, 6(8) (2011)
Link: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0023194
Forschungsteam: Wang, L., Li, C., Guo, H., Kern, T. S., Huang, K., Zheng, L.
Abstract:
Purpose: Neuron loss, glial activation, and vascular degeneration are common sequelae of ischemia-reperfusion (I/R) injury in ocular diseases. The present study was conducted to explore the ability of curcumin to inhibit retinal I/R injury and to investigate underlying mechanisms of the drug effects.
Methodology/Principal Findings: Different dosages of curcumin were administered. I/R injury was induced by elevating the intraocular pressure for 60 min followed by reperfusion. Cell bodies, brn3a-stained cells, and TUNEL-positive apoptotic cells in the ganglion cell layer (GCL) were quantitated, and the number of degenerate capillaries was assessed. The activation of glial cells was measured by the expression level of GFAP. Signaling pathways including IKK-IκBα, JAK-STAT1/3, ERK/MAPK, and the expression levels of β-tubulin III and MCP-1 were measured by western blot analysis. Pre-treatment using 0.01%-0.25% curcumin in diets significantly inhibited I/R-induced cell loss in GCL. 0.05% curcumin pre-treatment inhibited I/R-induced degeneration of retinal capillaries, TUNEL-positive apoptotic cell death in the GCL, brn3a-stained cell loss, the I/R-induced up-regulation of MCP-1, IKKα, p-IκBα and p-STAT3 (Tyr), and down-regulation of β-tubulin III. This dose showed no effect on injury-induced GFAP overexpression. Moreover, 0.05% curcumin administered 2 days after the injury also showed a vaso-protective effect.
Conclusions/Significance: Curcumin protects retinal neurons and microvessels against I/R injury. The beneficial effects of curcumin on neurovascular degeneration may occur through its inhibitory effects on injury-induced activation of NF-κB and STAT3, and on over-expression of MCP-1. Curcumin may therefore serve as a promising candidate for retinal ischemic diseases.
Veröffentlicht in: Brain Research, 1499, 145–157 (2013)
Link: https://www.sciencedirect.com/science/article/abs/pii/S0006899313000036?via%3Dihub
Forschungsteam: Koriyama, Y., Nakayama, Y., Matsugo, S., Kato, S.
Abstract: Oxidative stress plays a key role in neurodegeneration of CNS neurons such as in Alzheimer’s disease, Parkinson’s disease, and glaucoma. R-α-lipoic acid (R-LA) has been shown to have a neuroprotective effect through its antioxidant activity. However, the mechanism underlying its neuroprotection is totally unknown in retinal neurons. In this study, we show that R-LA has a dramatic neuroprotective effect against oxidative stress-induced death of the retinal neuronal RGC-5 cell line. We observed that R-LA induces the expression of heme oxygenase-1 (HO-1) by promoting the translocation of NF-E2-related factor 2 (Nrf2) to the nucleus. We examined the mechanism underlying HO-1 induction by R-LA by focusing on downstream signaling pathways. We found that R-LA activates Akt, and HO-1 induction by R-LA (involving Nrf2 translocation to the nucleus) was suppressed by phosphoinositide 3-kinase (PI3K) inhibitors. In addition, R-LA produced reactive oxygen species (ROS), including hydrogen peroxide. Pretreatment with a ROS scavenger or a NADPH oxidase inhibitor suppressed R-LA-induced Nrf2 translocation to the nucleus and HO-1 induction. These results suggest that ROS production triggered by R-LA might modify Kelch-like ECH-associated protein (Keap1), which in turn induces HO-1 expression through the PI3K signaling pathway. Furthermore, R-LA significantly attenuated cell death and accumulation of 4-hydroxy-2-nonenal (4HNE) in the retina induced by optic nerve injury in vivo through an HO-1 activity-dependent mechanism. These data demonstrate for the first time that R-LA exerts a neuroprotective effect against oxidative stress in retinal neurons in vitro and in vivo by inducing HO-1 through Keap1/Nrf2 signaling.
Veröffentlicht in: Biological and Pharmaceutical Bulletin, 36(7), 1060–1067 (2013)
Link: https://www.jstage.jst.go.jp/article/bpb/36/7/36_b12-00941/_article
Forschungsteam: Ji, D., Majid, A. S. A., Yin, Z. Q.
Abstract:
The aim of this study was to determine whether α-lipoic acid (LA) is effective in blunting the detrimental effect of light to transformed retinal ganglion cells (RGC-5 cells) in culture.
In this study, RGC-5 cells were exposed to light (400-760 nm; 1000 lx) for 48 h with or without LA. For cell assessment, 3-(4,5-di-methylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and 4-[3-(-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate (WST-1) reduction assays were used to assess cell and mitochondrial viability respectively. Furthermore, cells were stained for reactive oxygen species (ROS), apoptosis DNA breakdown, and apoptosis membrane alteration. Antioxidant capacity, glutathione (GSH), and glutathione-S-transferase (GST) were determined as well.
Light reduced cell viability, affected mitochondrial function, increased the number of terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL)-positive cells, and enhanced labeling for ROS. These effects were all attenuated by the presence of LA. LA also stimulated GSH and GST.
These findings support the view that light can affect mitochondria, which could lead to retinal ganglion cell apoptosis, and LA can blunt this by decreasing ROS generation and stimulating GSH and GST.
Veröffentlicht in: PLoS ONE, 11(8) (2016)
Link: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0160309
Forschungsteam: Wang, Y., Wang, W., Liu, J., Huang, X., Liu, R., Xia, H., Brecha, N. C., Pu, M., Gao, J.
Abstract: In this study, we first sought to determine whether RNA-binding protein with multiple splicing (RBPMS) can serve as a specific marker for cat retinal ganglion cells (RGCs) using retrograde labeling and immunohistochemistry staining. RBPMS was then used as an RGC marker to study RGC survival after optic nerve crush (ONC) and alpha-lipoic acid (ALA) treatment in cats. ALA treatment yielded a peak density of RBPMS-alpha cells within the peak isodensity zone (>60/mm²), which did not differ from ONC retinas. The area within the zone was significantly enlarged (control: 2.3%, ONC: 0.06%, ONC+ALA: 0.1%). As for the 10-21/mm² zone, ALA treatment resulted in a significant increase in area (control: 34.5%, ONC: 12.1%, ONC+ALA: 35.9%). ALA can alleviate crush-induced RGC injury.
Veröffentlicht in: PLoS ONE, 8(6) (2013)
Link: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0065389
Forschungsteam: Inman, D. M., Lambert, W. S., Calkins, D. J., Horner, P. J..
Abstract: Oxidative stress has been implicated in neurodegenerative diseases, including glaucoma. However, due to the lack of clinically relevant models and the expense of long-term testing, few studies have modeled antioxidant therapy for the prevention of neurodegeneration. We investigated the contribution of oxidative stress to the pathogenesis of glaucoma in the DBA/2J mouse model of glaucoma. Similar to other neurodegenerative diseases, we observed lipid peroxidation and upregulation of oxidative stress-related mRNA and protein in DBA/2J retina. To test the role of oxidative stress in disease progression, we chose to deliver the naturally occurring antioxidant α-lipoic acid (ALA) to DBA/2J mice in their diet. We used two paradigms for ALA delivery: an intervention paradigm in which DBA/2J mice at 6 months of age received ALA in order to intervene in glaucoma development, and a prevention paradigm in which DBA/2J mice were raised on a diet supplemented with ALA, with the goal of preventing glaucoma development. At 10 and 12 months of age (after 4 and 11 months of dietary ALA, respectively), we measured changes in genes and proteins related to oxidative stress, retinal ganglion cell (RGC) number, axon transport, and axon number and integrity. Both ALA treatment paradigms showed increased antioxidant gene and protein expression, increased protection of RGCs, and improved retrograde transport compared to control. Measures of lipid peroxidation, protein nitrosylation, and DNA oxidation in retina verified decreased oxidative stress in the prevention and intervention paradigms. These data demonstrate the utility of dietary therapy for reducing oxidative stress and improving RGC survival in glaucoma.
Veröffentlicht in: Journal of Neuroendocrinology, 30(1) (2018)
Link: https://onlinelibrary.wiley.com/doi/10.1111/jne.12567id=10.1371/journal.pone.0065389
Forschungsteam: Kim, J. H., Choi, B. Y., Kho, A. R., Lee, S. H., Jeong, J. H., Hong, D. K., Lee, S. H., Sohn, M., Ryu, O. H., Choi, M.-G., Suh, S. W.
Abstract: Citicoline (cytidine 5′-diphosphocholine) is an important precursor for the synthesis of neuronal plasma membrane phospholipids, mainly phosphatidylcholine. The administration of citicoline serves as a choline donor for the synthesis of acetylcholine. Citicoline has been shown to reduce neuronal injury in animal models with cerebral ischemia and in clinical trials of stroke patients. Citicoline is currently being investigated in a multicenter clinical trial. However, citicoline has not yet been examined in the context of hypoglycemia-induced neuronal death. To clarify the therapeutic impact of citicoline in hypoglycemia-induced neuronal death, we used a rat model with insulin-induced hypoglycemia. Acute hypoglycemia was induced by i.p. injection of regular insulin (10 U/kg) after overnight fasting, after which iso-electricity was maintained for 30 minutes. Citicoline injections (500 mg/kg, i.p.) were started immediately after glucose reperfusion. We found that post-treatment of citicoline resulted in significantly reduced neuronal death, oxidative injury, and microglial activation in the hippocampus compared to vehicle-treated control groups at 7 days after induced hypoglycemia. Citicoline administration after hypoglycemia decreased immunoglobulin leakage via blood-brain barrier disruption in the hippocampus compared to the vehicle group. Citicoline increased choline acetyltransferase expression for phosphatidylcholine synthesis after hypoglycemia. Altogether, the present findings suggest that neuronal membrane stabilization by citicoline administration can save neurons from the degeneration process after hypoglycemia, as seen in several studies of ischemia. Therefore, the results suggest that citicoline may have therapeutic potential to reduce hypoglycemia-induced neuronal death.
Veröffentlicht in: Brain Research, 1595, 156–165 (2015)
Link: https://www.sciencedirect.com/science/article/abs/pii/S0006899314015583?via%3Dihub
Forschungsteam: Kim, J. H., Lee, D. W., Choi, B. Y., Sohn, M., Lee, S. H., Choi, H. C., Ki Song, H., Suh, S. W.
Abstract: Citicoline (CDP-choline; cytidine 5′-diphosphocholine) is an important intermediate in the biosynthesis of cell membrane phospholipids. Citicoline serves as a choline donor in the biosynthetic pathways of acetylcholine and neuronal membrane phospholipids, mainly phosphatidylcholine. The ability of citicoline to reverse neuronal injury has been tested in animal models of cerebral ischemia, and clinical trials have been performed in stroke patients. However, no studies have examined the effect of citicoline on seizure-induced neuronal death. To clarify the potential therapeutic effects of citicoline on seizure-induced neuronal death, we used an animal model of pilocarpine-induced epilepsy. Temporal lobe epilepsy (TLE) was induced by intraperitoneal injection of pilocarpine (25 mg/kg) in adult male rats. Citicoline (100 or 300 mg/kg) was injected into the intraperitoneal space two hours after seizure onset, and a second injection was performed 24 hours after the seizure. Citicoline was injected once per day for one week after pilocarpine- or kainate-induced seizure. Neuronal injury and microglial activation were evaluated at 1 week post-seizure. Surprisingly, rather than offering protection, citicoline treatment actually enhanced seizure-induced neuronal death and microglial activation in the hippocampus compared to vehicle-treated controls. Citicoline administration after seizure induction increased immunoglobulin leakage via BBB disruption in the hippocampus compared with the vehicle-only group. To clarify if this adverse effect of citicoline is generalizable across alternative seizure models, we induced seizure by kainate injection (10 mg/kg, i.p.) and then injected citicoline as in pilocarpine-induced seizure. We found that citicoline did not modulate kainate seizure-induced neuronal death, BBB disruption, or microglial activation. These results suggest that citicoline may not have neuroprotective effects after seizure and that clinical application of citicoline after seizure needs careful consideration.
Veröffentlicht in: Journal of Biochemical and Molecular Toxicology, 18(5), 273–278 (2004)
Link: https://onlinelibrary.wiley.com/doi/10.1002/jbt.20037
Forschungsteam: Aabdallah, D. M., Eid, N. I.
Abstract: A close correlation exists between ischemia/reperfusion (I/R)-induced insult and the release of free radicals. Lecithin is a polyunsaturated phosphatidylcholine that corresponds to the phosphatidylcholine molecule. Phosphatidylcholines are high-energy functional and structural elements of all biologic membranes. α-Tocopherol is the major lipid-soluble chain-breaking antioxidant in body tissues and effectively protects against neuronal damage. Therefore, we studied the effect of lecithin (300 mg/kg, P.O., 14 days) and α-tocopherol (200 mg/kg, P.O., 14 days), alone or in combination, on the brain redox state during I/R. Adult male Wistar rats were subjected to global ischemia by the occlusion of the two carotid arteries 24 hours after the last dose of drug administration. Reperfusion was carried out 1 hour after the induction of ischemia and lasted for another hour. Brain lipid peroxides (MDA) and glutathione (GSH) contents, as well as superoxide dismutase (SOD) and catalase (CAT) activities, were assessed. The results showed that I/R elevated brain lipid peroxides content, which was accompanied by a reduction in both antioxidant enzyme activities; however, brain GSH levels remained unaltered. Lecithin, α-tocopherol, and their combination restored MDA content, as well as CAT activity, with a slight tendency to normalize SOD activity. We conclude that lecithin has a possible neuroprotective effect partly through its antioxidant action, which is comparable to that of α-tocopherol.
Veröffentlicht in: Brain Research, 1226, 226–233 (2008)
Link: https://www.sciencedirect.com/science/article/abs/pii/S0006899308014327?via%3Dihub
Forschungsteam: Nakajima, Y., Inokuchi, Y., Nishi, M., Shimazawa, M., Otsubo, K., Hara, H
Abstract:
Purpose: To investigate the neuroprotective effects of coenzyme Q10 and/or a vitamin E analogue on retinal damage both in vitro and in vivo.
Methods: We employed cultured retinal ganglion cells (RGC-5, a rat ganglion cell-line transformed using E1A virus) in vitro. Cell damage was induced by 24-hour hydrogen peroxide (H2O2) exposure, and cell viability was measured using tetrazolium salt (WST-8). To examine the retinal damage induced by intravitreal N-methyl-d-aspartate (NMDA) injection in mice in vivo, coenzyme Q10 at 10 mg/kg with or without alpha-tocopherol at 10 mg/kg was administered orally each day for 14 days, with NMDA being intravitreally injected on day 7 of this course.
Results: In RGC-5, a combination of coenzyme Q10 and trolox, a water-soluble vitamin E analogue (a derivative of alpha-tocopherol), prevented cell damage more effectively than either agent alone. Coenzyme Q10 and alpha-tocopherol (separately or together) reduced the retinal damage, number of TUNEL-positive cells in the ganglion cell layer (GCL), and 4-hydroxyl-2-nonenal (4-HNE) expression induced by NMDA in mice in vivo.
Conclusions: Coenzyme Q10 and/or these vitamin E analogues exert neuroprotective effects against retinal damage both in vitro and in vivo.
Veröffentlicht in: Investigative Ophthalmology and Visual Science, 55(2), 993–1005 (2014)
Link: https://iovs.arvojournals.org/article.aspx?articleid=2190253
Forschungsteam: Lee, D., Shim, M. S., Kim, K. Y., Noh, Y. H., Kim, H., Kim, S. Y., Weinreb, R. N., Ju, W. K.
Abstract:
Purpose: To test whether a diet supplemented with coenzyme Q10 (CoQ10) ameliorates glutamate excitotoxicity and oxidative stress-mediated retinal ganglion cell (RGC) degeneration by preventing mitochondrial alterations in the retina of glaucomatous DBA/2J mice.
Methods: Preglaucomatous DBA/2J and age-matched control DBA/2JGpnmb+ mice were fed with CoQ10 (1%) or a control diet daily for 6 months. RGC survival and axon preservation were measured by Brn3a and neurofilament immunohistochemistry and by conventional transmission electron microscopy. Glial fibrillary acidic protein (GFAP), superoxide dismutase-2 (SOD2), heme oxygenase-1 (HO1), N-methyl-D-aspartate receptor (NR) 1 and 2A, and Bax and phosphorylated Bad (pBad) protein expression were measured by Western blot analysis. Apoptotic cell death was assessed by TUNEL staining. Mitochondrial DNA (mtDNA) content and mitochondrial transcription factor A (Tfam)/oxidative phosphorylation (OXPHOS) complex IV protein expression were measured by real-time PCR and Western blot analysis.
Results: Coenzyme Q10 promoted RGC survival by approximately 29% and preserved the axons in the optic nerve head (ONH), as well as inhibited astroglial activation by decreasing GFAP expression in the retina and ONH of glaucomatous DBA/2J mice. Intriguingly, CoQ10 significantly blocked the upregulation of NR1 and NR2A, as well as of SOD2 and HO1 protein expression in the retina of glaucomatous DBA/2J mice. In addition, CoQ10 significantly prevented apoptotic cell death by decreasing Bax protein expression and increasing pBad protein expression. More importantly, CoQ10 preserved mtDNA content and Tfam/OXPHOS complex IV protein expression in the retina of glaucomatous DBA/2J mice.
Conclusions: Our findings suggest that CoQ10 may be a promising therapeutic strategy for ameliorating glutamate excitotoxicity and oxidative stress in glaucomatous neurodegeneration.
Veröffentlicht in: Mitochondrion, 36, 114–123 (2017)
Link: https://www.sciencedirect.com/science/article/pii/S1567724917301423?via%3Dihub
Forschungsteam: Davis, B. M., Tian, K., Pahlitzsch, M., Brenton, J., Ravindran, N., Butt, G., Malaguarnera, G., Normando, E. M., Guo, L., Cordeiro, M. F.
Abstract: Coenzyme Q10 (CoQ10) is a mitochondrial-targeted antioxidant with known neuroprotective activity. Its ocular effects when co-solubilized with α-tocopherol polyethylene glycol succinate (TPGS) were evaluated. In vitro studies confirmed that CoQ10 was significantly protective in different retinal ganglion cell (RGC) models. In vivo studies in Adult Dark Agouti (DA) rats with unilateral surgically-induced ocular hypertension (OHT) treated with either CoQ10/TPGS micelles or TPGS vehicle twice daily for three weeks were performed, following which retinal cell health was assessed in vivo using DARC (Detection of Apoptotic Retinal Cells) and post-mortem with Brn3a histological assessment on whole retinal mounts. CoQ10/TPGS showed a significant neuroprotective effect compared to control with DARC (p < 0.05) and Brn3a (p < 0.01). Topical CoQ10 appears to be an effective therapy for preventing RGC apoptosis and loss in glaucoma-related models.
Veröffentlicht in: nternational Ophthalmology, 25(5–6), 283–289 (2004)
Link: https://link.springer.com/article/10.1007/s10792-005-2034-z
Forschungsteam: Aydemir, O., Çelebi, S., Yılmaz, T., Yekeler, H., Kükner, A. Ş.
Abstract:
Purpose: The purpose of this study is to provide evidence that free radical damage is a component of retinal ischemia–reperfusion (I/R) injury, and to determine whether alpha-tocopherol, gamma-tocopherol, and d-alpha-tocopherol polyethylene glycol 1000 succinate (TPGS) can protect the retina from this injury.
Methods: The right eyes of 40 male guinea pigs weighing 500–600 g were used. The animals were randomly assigned to group 1 (control), group 2 (I/R), group 3 (I/R plus alpha-tocopherol), group 4 (I/R plus gamma-tocopherol), and group 5 (I/R plus TPGS). Groups 3, 4, and 5 received four subcutaneous injections at six-hour intervals for a total dosage of 800 IU/kg alpha-tocopherol, 1000 IU/kg gamma-tocopherol, and 750 IU/kg TPGS, respectively. The first dose of each substance was administered 5 minutes before retinal ischemia. Retinal ischemia was induced for 90 minutes, followed by reperfusion for 24 hours. Injections of the three substances were repeated at 6, 12, and 18 hours during reperfusion. The animals were killed at 24 hours of reperfusion. Sagittal sections of 4 μm were cut and stained with hematoxylin and eosin for light microscopic evaluation. The average thickness (edema) of the inner plexiform layer for each eye was measured in sagittal sections near the optic nerve and expressed in microns.
Results: All three substances showed statistically significant protection against the formation of retinal edema during ischemia–reperfusion injury. The mean thickness of the inner plexiform layer was 15.0, 25.44, 19.81, 21.38, and 20.88 μm in control, I/R, I/R plus alpha-tocopherol, I/R plus gamma-tocopherol, and I/R plus TPGS groups, respectively. The results showed that the thickness of the inner plexiform layer in group 1 (control) was significantly lower than the other groups (p<0.001). The inner plexiform layer was thicker in the I/R group than in the I/R plus alpha-tocopherol (p<0.001), I/R plus gamma-tocopherol (p<0.001), and I/R plus TPGS (p<0.01) groups. The inner plexiform layer was not thicker in the I/R plus TPGS group than in the I/R plus alpha-tocopherol and I/R plus gamma-tocopherol groups. Compared to the I/R plus alpha-tocopherol group, the inner plexiform layer was significantly thicker in the I/R plus gamma-tocopherol group (p<0.01).
Conclusions: The results from these experiments indicate that vitamin E forms have protective effects on the retina during retinal ischemia-reperfusion injury, but the effects of alpha-tocopherol and TPGS appear to be much greater than that of gamma-tocopherol.
Veröffentlicht in: Life Science Alliance, 6(8) (2023)
Link: https://www.life-science-alliance.org/content/6/8/e202301976
Forschungsteam: Li, S., Jakobs, T. C.
Abstract: Glaucoma is a common neurodegenerative disorder characterized by retinal ganglion cell death, astrocyte reactivity in the optic nerve, and vision loss. Currently, lowering intraocular pressure (IOP) is the first-line treatment, but adjuvant neuroprotective approaches would be welcome. Vitamin C possesses neuroprotective activities that are thought to be related to its properties as a co-factor of enzymes and its antioxidant effects. Here, we show that vitamin C promotes a neuroprotective phenotype and increases gene expression related to neurotropic factors, phagocytosis, and mitochondrial ATP production. This effect is dependent on the up-regulation of secreted phosphoprotein 1 (SPP1) in reactive astrocytes via the transcription factor E2F1. SPP1+ astrocytes in turn promote retinal ganglion cell survival in a mouse model of glaucoma. In addition, oral administration of vitamin C lowers the IOP in mice. This study identifies an additional neuroprotective pathway for vitamin C and suggests a potential therapeutic role of vitamin C in neurodegenerative diseases such as glaucoma.
Veröffentlicht in: Nutrients, 12(4) (2020)
Link: https://www.mdpi.com/2072-6643/12/4/1189
Forschungsteam: Cammalleri, M., Monte, M. D., Amato, R., Bagnoli, P., Rusciano, D.
Abstract: There is indication that nutritional supplements protect retinal cells from degeneration. In a previous study, we demonstrated that dietary supplementation with a combination of forskolin, homotaurine, spearmint extract, and B vitamins efficiently counteracts retinal dysfunction associated with retinal ganglion cell (RGC) death caused by optic nerve crush. We extended our investigation on the efficacy of dietary supplementation using a mouse model in which RGC degeneration depends as closely as possible on intraocular pressure (IOP) elevation. In this model, injecting the anterior chamber of the eye with methylcellulose (MCE) causes IOP elevation leading to RGC dysfunction. The MCE model was characterized in terms of IOP elevation, retinal dysfunction as determined by electrophysiological recordings, RGC loss as determined by brain-specific homeobox/POU domain protein 3A immunoreactivity, and dysregulated levels of inflammatory and apoptotic markers. Except for IOP elevation, dysfunctional retinal parameters were all recovered by dietary supplementation, indicating the involvement of non-IOP-related neuroprotective mechanisms of action. Our hypothesis is that the diet supplement may counteract the inflammatory processes triggered by glial cell activation, thus leading to spared RGC loss and the preservation of visual function. In this respect, the present compound may be viewed as a potential remedy to be added to the currently approved drug therapies for improving RGC protection.
Veröffentlicht in: Journal of Ocular Pharmacology and Therapeutics, 32(3), 178–183 (2016)
Link: https://www.liebertpub.com/doi/10.1089/jop.2015.0121
Forschungsteam: Mutolo, M. G., Albanese, G., Rusciano, D., Pescosolido, N.
Abstract:
Purpose: To evaluate the effects of a food supplement containing forskolin, homotaurine, carnosine, folic acid, vitamins B1, B2, B6, and magnesium in patients with primary open angle glaucoma (POAG) already in treatment and compensated by intraocular pressure (IOP)-lowering drugs, during a period of 12 months.
Methods: Twenty-two patients (44 eyes) with POAG, with their IOP compensated by topical drugs, were enrolled and randomly assigned to the food supplement or control treatment group. The additional food supplement treatment consisted of 2 tablets per day (1 in the morning, 1 in the evening) given for 1 year of a balanced association of homotaurine, Coleus forskohlii root extract, l-carnosine, folic acid, vitamins B1, B2, B6, and magnesium. Pattern Electroretinogram (PERG) amplitude, foveal sensitivity obtained with the visual field analyzer frequency doubling technology, and IOP were detected at enrollment (T0), 3 months (T1), 6 months (T2), 9 months (T3), and 12 months (T4).
Results: We observed in treated patients a significant further decrease of IOP and an improvement of PERG amplitude at 6, 9, and 12 months, and foveal sensitivity at 12 months. All values remained substantially stable in control patients.
Conclusions: The results of the present pilot study indicate that the components of the food supplement reach the eye in a detectable manner, as evidenced by the effects on the IOP. Moreover, they suggest a short-term neuroactive effect, as indicated by the improvement of PERG amplitude and foveal sensitivity in treated, but not in control patients.
Veröffentlicht in: Journal of Ophthalmology (2020)
Link: https://onlinelibrary.wiley.com/doi/10.1155/2020/7879436
Forschungsteam: Rolle, T., Dallorto, L., Rossatto, S., Curto, D., Nuzzi, R.
Abstract:
Background: Glaucoma is a multifactorial optic neuropathy, which causes a continuous loss of retinal ganglion cells. Given the neurodegenerative nature of glaucoma, the necessity for neuroprotective intervention still arises, to be added alongside hypotonic therapy.
Objective: The objective of this study was to assess the effect of daily intake of a homotaurine, carnosine, forskolin, vitamins B1, B2, and B6, folic acid, and magnesium based supplement (GANGLIOLIFE®) on the progression rates of the visual field in patients with progressive POAG despite good tonometric compensation and to assess the most suitable dosage.
Methods: This is a monocentric nonrandomized experimental clinical study. Patients with mean deviation (MD) ranging from −2 dB to −15 dB with MD progression ≥1 dB in the previous year and IOP values of ≤18 mm Hg were included. All the patients underwent supplement therapy for a period of 6 months. For the first 2 months, they took 2 tablets a day, and for the following 4 months, 1 tablet a day. The patients were assessed before the start of treatment, time 0 (T0), after 2 months (T1), and after 6 months (T2) of therapy. At each check-up, patients were given a full eye test including perimetry, RNFL, and GCC using FD-OCT, PERG, contrast sensitivity, and QoL evaluation using the Glaucoma Symptom Scale questionnaire and National Eye Institute Visual Function Questionnaire 25.
Results: 31 patients with a mean age of 70.80 ± 8.77 were included. At T1 and T2, the mean values of MD were lessened (MD = −5.37 ± −2.91, P < 0.01, and MD = −5.48 ± 3.15, P < 0.05, respectively) compared to T0 (MD = -5.98 ± 2.83). Patients also demonstrated a significant reduction in IOP (P < 0.01), improved light sensitivity (P < 0.01) and contrast sensitivity (P < 0.05), and a better quality of life (P < 0.05).
Conclusions: Treatment with a supplement which includes homotaurine, carnosine, forskolin, vitamins B1, B2, and B6, folic acid, and magnesium has been shown to be able to slow down the rate of progression of functional damage and improve visual function after 2 and 6 months of daily intake. Quality of life showed significant improvement.
Veröffentlicht in: Ophthalmologica, 191(4), 238–249 (1985)
Forschungsteam: Stark, H.
Abstract: Investigations on 114 glaucoma patients with the computerized perimeter ‘Peritest’ showed that treatment with Cosaldon A + E resulted in a decrease or elimination of visual field defects. Favourable results were also obtained with statistical significance for severe visual field defects of defect class≥2.0 logU. Freatment time was generally 4–5 months. During the first 6–10 weeks of treatment strong fluctuations were observed regarding individual sensitivity of light perception as well as number of defect positions.
Veröffentlicht in: Acta Ophthalmologica Scandinavica, 76(S227), 41–42 (1998)
Link: https://onlinelibrary.wiley.com/doi/10.1111/j.1600-0420.1998.tb00880.x
Forschungsteam: Cellini, M., Caramazza, N., Mangiafico, P., Possati, G. L., Caramazza, R.
Summary:
The authors have studied the efficacy of an association of DHA, vitamin E and vitamin B complex (TROFINERV) in glaucomatous patients.
The parameters evaluated were computerized visual field (CVF) and retinal contrast sensitivity (RCS).
Thirty chronic simple glaucoma patients in good tensional compensation with local therapy were given TROFINERV oral therapy. The results show significant differences after 90 days of treatment both in the perimetric indices (MD, SF, CPSD; p<0.05) and in the RCS frequency values (p<0.05).
In the light of the results obtained, the authors consider the use of such association to be a useful support in glaucomatous patient therapy for preventing or delaying the progress of damage.
Veröffentlicht in: European Journal of Ophthalmology, 17(4), 528–533 (2007)
Link: https://journals.sagepub.com/doi/10.1177/112067210701700408
Forschungsteam: Engin, K. N., Engin, G., Kucuksahin, H., Oncu, M., Engin, G., Guvener, B.
Abstract:
Purpose: To evaluate the vasoregulatory effect of antioxidant alpha-tocopherol on retina via protein kinase C pathway.
Methods: Thirty glaucomatous patients (60 eyes) were included in this study. The patients were divided into three groups. For patients in Group A, tocopherol was not supplemented in their therapy. Patients in Groups B and C received 300 and 600 mg/day of oral alpha-tocopherol acetate, respectively. The ultimate blood tocopherol levels were confirmed via high-performance liquid chromatography assay. Progression of the disease for each subject was monitored via visual field measurements and color Doppler imaging of ophthalmic and posterior ciliary arteries at the beginning and at the 6th and 12th months of this study.
Results: The average differences between the pulsatility indexes (PI) and resistivity indexes (RI) of both ophthalmic arteries (OA) and posterior ciliary arteries (PCA) of Groups B and C were significantly lower than those of Group A at months 6 and 12. In trial groups, RI decreases observed in PCAs at months 6 and 12 and PI decreases observed in OAs at the 6th month were statistically significant. Differences of mean deviations with visual fields in Groups B and C were highly significantly lower than that of Group A.
Conclusions: Alpha-tocopherol deserves attention beyond its antioxidant properties for protecting retina from glaucomatous damage.
Veröffentlicht in: European Journal of Ophthalmology, 17(4), 528–533 (2007)
Link: https://www.mediasphera.ru/issues/vestnik-oftalmologii/2013/2/downloads/ru/030042-465X2013215
Forschungsteam: Dolgova, I. G., Malishevskaia, T. N., Shatskikh, S. V, Lazareva, T. P., Ampilova, T. P., Nemtsova, I. V, Dorkina, I. L., Antipina, N. A., Kalinina, O. N.
Abstract: The efficacy of Focus forte is proved in patients with primary open-angle glaucoma (POAG) and age-related macular degeneration (AMD) in terms of improvement of functional retinal activity, oxygenation, metabolism normalization as well as morphometric retinal and optic nerve indices. Principles of evidence based pharmacotherapy in this study allow advising Focus forte in the treatment of patients with POAG and AMD.
Veröffentlicht in: Acta Ophthalmologica, 96(2), e237–e241 (2018)
Link: https://www.mediasphera.ru/issues/vestnik-oftalmologii/2013/2/downloads/ru/030042-465X2013215
Forschungsteam: Harris, A., Gross, J., Moore, N., Do, T., Huang, A., Gama, W., Siesky, B.
Abstract:
Purpose: To investigate the effects of an antioxidant dietary supplement that includes Ginkgo biloba, on retinal and retrobulbar blood flow in patients with open-angle glaucoma (OAG).
Methods: Forty-five patients with confirmed OAG were enroled in a randomized, double blinded, placebo-controlled cross-over study. Baseline and postadministration measurements of intraocular pressure (IOP), ocular perfusion pressure (OPP), retrobulbar blood flow, and retinal capillary blood flow were non-invasively measured (ultrasound and laser Doppler modalities, respectively) before and one month after antioxidant nutraceuticals and placebo administration. Changes in measurements between the active supplement and placebo arms were evaluated using paired t-tests, with p < 0.05 considered statistically significant.
Results: Antioxidant supplementation produced a statistically significant increase in peak systolic and/or end diastolic blood flow velocities in all retrobulbar blood vessels compared to placebo. Vascular resistance was also reduced in central retinal and nasal short posterior ciliary arteries following antioxidant administration. Additionally, antioxidant supplementation increased superior and inferior temporal retinal capillary mean blood flow and the ratio of active to non-active retina capillaries compared to placebo.
Conclusion: One-month oral administration of antioxidants produced increases in biomarkers of ocular blood flow within retinal and retrobulbar vascular beds in patients with OAG.
Veröffentlicht in: Archivos de La Sociedad Española de Oftalmología (English Edition), 95(3), 120–129 (2020)
Link: https://www.sciencedirect.com/science/article/abs/pii/S0365669119303533?via%3Dihub
Forschungsteam: Sanz-González, S. M., Raga-Cervera, J., Aguirre Lipperheide, M., Zanón-Moreno, V., Chiner, V., Ramírez, A. I., Pinazo-Durán, M. D.
Abstract:
Objective: To analyse the safety and effectiveness of the oral administration of a commercialised supplement containing R-alpha lipoic acid, taurine, vitamins C and E, lutein, zeaxanthin, zinc, copper and docosahexaenoic acid, in patients with primary open angle glaucoma (POAG), and in control subjects.
Material and methods: A prospective study of cases and controls was carried out, including 30 participants of both genders that were divided into: POAG Group (n = 15) and a control group (CG; n = 15), assigned to the oral intake of NuaDHA preparations Vision® (1 pill/day) + NuaDHA 1000 (2 pills/day) for 6 months. Participants were interviewed, ophthalmologically examined, and peripheral blood was taken for routine analysis and the determination of the pro-oxidant (malondialdehyde) and total antioxidant status. Statistical analysis was performed using the SPSS 22.0 program.
Results: After 6 months of supplementation, there was a significant increase in the plasma total antioxidant status (1.073 ± 0.090 mM vs 1.276 ± 0.107 mM, P = .028), along with a parallel decrease in malondialdehyde (7.066 ± 1.070 μM vs 2.771 ± 0.462 μM, P = .005) in the POAG group. The malondialdehyde also decreased in the control group (6.17 ± 1.336 vs. 2.51 ± 0.391, P = .028). The Schirmer test improved (20-30%) and the subjective dry eye signs/symptoms noticeably decreased in the POAG group versus the CG.
Conclusions: Formulations containing antioxidant vitamins, R-alpha lipoic acid and docosahexaenoic acid, administered for 6 consecutive months, counteracted the oxidative stress by further stabilising the morphological/functional parameters of both the ocular surface and the glaucoma, without presenting with adverse effects or intolerances.
Dieser Beitrag ist ausschließlich für medizinisches Fachpersonal bestimmt. Bitte bestätigen Sie unten: